A Theoretical Basis for a Biopharmaceutics Drug Classification. Davit, June 2011, 'BCS Classification Workshop, Canadian Society for. Specified List. Davit, June 2011, ‘BCS Classification Workshop, Canadian Society for Pharmaceutical Sciences ‘FDA Experience with Biopharmaceutics Classification System (BCS) Biowaivers New Drugs vs. Generics Between 2003 – June, 2011, 54 drug products submitted to FDA for classification. Our resources page contains links to presentations, posters, abstracts, and links to the Drug Delivery Foundation's BCS database. Crack photoshop cc 2015. Microsoft office 2013 activator download.
Views 22,322
From Embeds
Bcs Classification List Of Drugs Pdf
Number of Embeds
 Actions
ActionsDownloads
Comments
Likes
Bcs Classification List
Embeds 0
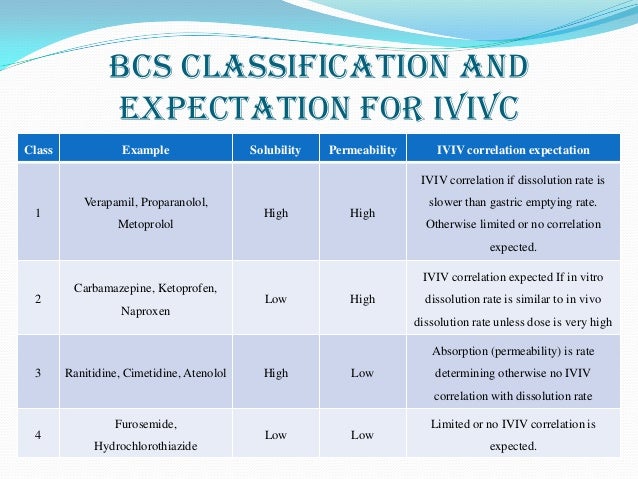
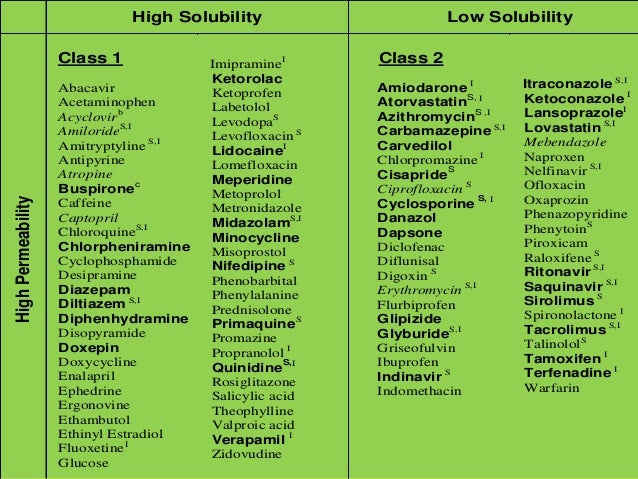
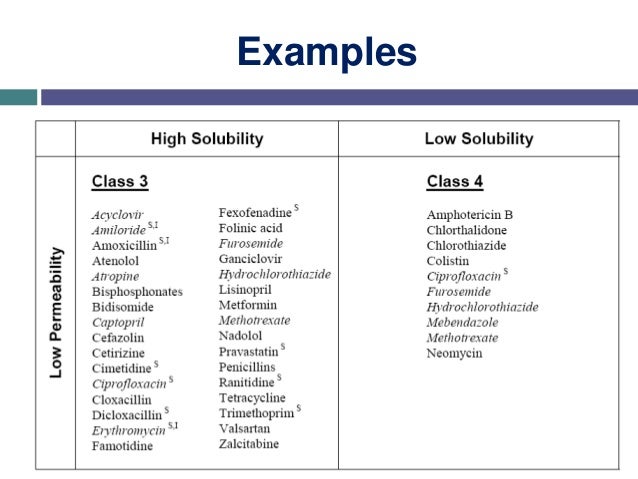
Biopharmaceutical Classification System (BCS) is a scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability
when the dissolution rate is much greater than the gastric emptying, dissolution is not likely to be rate-limiting
the pharmaceutical industry